Cathodic Protection
What is Cathodic Protection?
Cathodic protection ( CP ) is a system/ technique used to control or mitigate corrosion damage to metal surfaces, by making it the Cathodic side of an Electrochemical cell. It can be used to protect:

Cathodic Protection System
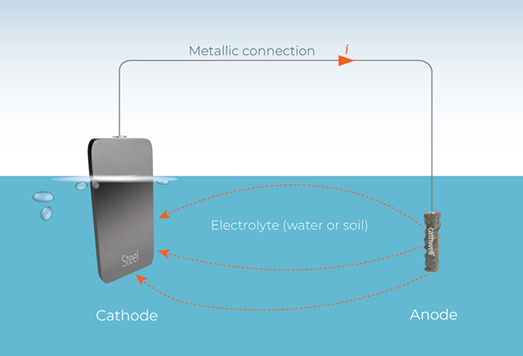
A Cathodic Protection System can be used to protect steel from corrosion. Corrosion is caused when two different metals are submerged in an electrolytic substance such as water, concrete or soil. Corrosion is the action of a metal that has been extracted from ore, reverting back to its original or primary state , when exposed to oxygen and water/ moisture.
This type of metal conducting path between the two different metals allows a pathway through which any free electrons can move from the more active metal (anode) to the less active metal (cathode). If free electrons from the anode do not reach active sites on the cathode before the arrival of oxygen, ions at the active sites can then recombine to produce ferrous hydroxide, i.e. rust
How does a cathodic protection work?
Basically ,Cathodic protection connects the base metal at risk (steel) to a sacrificial metal that corrodes in lieu of the base metal. This technique of providing Cathodic protection to steel, assists in preserving the metal, by providing a highly active metal, that can act as an anode and provide free electrons. By introducing these free electrons, the active metal sacrifices its ions and keeps the less active steel from corroding.
Two basic types of cathodic protection

Galvanic Protection
Galvanic Protection consists of applying a layer of protective zinc coating to the steel to prevent rusting / corrosion. The zinc corrodes in place of the encapsulated steel. These systems can have a limited life span. The Sacrificial anode protecting the metal will continue to degrade over time, until the Sacrificial anode is no longer capable of supplying protection.
Impressed current cathodic protection
Impressed Current Cathodic Protection systems, consist of anodes that are connected to a power source that provides the required electrical flow. The Sacrificial anode method of protection uses, a metal more active than the base metal to “sacrifice” ions. These “Sacrificial anodes” (usual alloys such as magnesium, aluminum, or zinc) have a stronger electrochemical potential. This method can often provide much longer protection than a Galvanic anode as the anode is supplied by an unlimited or constant power source.

